Practice Insights: Emerging Insulins
In 2016, APhA conducted a survey to explore pharmacists’ current diabetes management activities in community settings and assess pharmacists’ familiarity with emerging insulin products. This visually-enhanced, statistical report summarizes the findings – obtained from interviews, discussion groups and survey results.
- Download the full report. (PDF)
The American Pharmacists Association (APhA) gratefully acknowledges the following individuals who served as content reviewers and pharmacy practice advisors:
Jennifer D. Smith, PharmD
Associate Professor
Campbell University College of Pharmacy & Health Sciences
Clinical Pharmacist Practitioner Wilson Community Health Center
Staci-Marie Norman, PharmD
Clinical Coordinator Martin’s Pharmacy
This report and associated survey were supported by Sanofi as part of a collaboration with APhA. Sanofi provided comments; however, APhA retained full editorial control over the survey and final content.
Introduction
Diabetes is a prevalent and costly disease that is challenging to manage. There were approximately 29 million individuals in the United States with diabetes in 2014, and the annual costs of diabetes were estimated to be $245 billion in 2012.1,2 Diabetes is a complex and multifactorial disease that requires patients to take an active self-management role to prevent both acute and long-term complications.
A large body of research has demonstrated that pharmacists’ delivery of patient care services can improve outcomes for patients with diabetes. A systematic review of 36 studies that evaluated the effectiveness of pharmacists’ interventions for improving outcomes found numerous beneficial effects. These effects included improvements in A1C, blood pressure, cholesterol levels, body mass index, and 10-year cardiovascular risk.3 Pharmacists also were found to significantly improve patients’ medication adherence and health-related quality of life. All studies that assessed financial outcomes found that pharmacists’ services were cost effective.3
In current pharmacy practice, pharmacists in diverse settings provide a wide range of services for patients with diabetes to support them with managing their conditions and addressing their health care needs. The continuum of services includes activities such as:
- Medication counseling.
- Education in diabetes self-care skills.
- Targeted pharmacy-based services such as blood glucose meter training, foot care, wound care, etc.
- Comprehensive diabetes self-management education.
- Protocol-driven medication management under collaborative practice agreements.
- Independent direct patient care.
To explore pharmacists’ current diabetes management activities in community settings and assess pharmacists’ familiarity with emerging insulin products, the American Pharmacists Association (APhA) conducted a survey in 2016. A total of 330 pharmacists responded to the online portion of the survey and an additional 10 pharmacists participated in in-depth telephone interviews.
Survey respondents practiced in a variety of community pharmacy settings (31% chain pharmacy, 27% independent pharmacy, 20% supermarket pharmacy, 10% ambulatory care clinic, 6% mass-merchant pharmacy, and 6% other). Respondents reported that they fill a mean of 309.5 prescriptions per day, and 74.5% of their professional practice activities are devoted to direct patient care (including prescription processing).
Provision of Patient Care Services for Diabetes
 Pharmacists responding to the APhA survey reported providing a variety of services for patients with diabetes. A subset of survey respondents (20%) operate formal diabetes management programs in their practice. While pharmacists with such programs were more likely to provide in-depth patient care services, pharmacists without formal programs also provide a wide range of diabetes-related services. The pharmacists also reported using a variety of documentation strategies to support their services.
Pharmacists responding to the APhA survey reported providing a variety of services for patients with diabetes. A subset of survey respondents (20%) operate formal diabetes management programs in their practice. While pharmacists with such programs were more likely to provide in-depth patient care services, pharmacists without formal programs also provide a wide range of diabetes-related services. The pharmacists also reported using a variety of documentation strategies to support their services.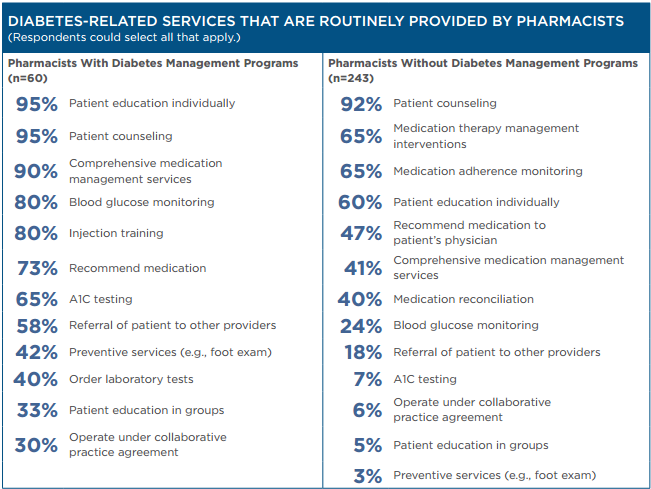
Pharmacists’ Patient Education Activities
It is well-established that tight glycemic control prevents the development of both microvascular and macrovascular complications of diabetes. However, close to half of all patients do not achieve the glycemic goal established by the American
Diabetes Association (ADA) of an A1C <7%.4-6
To achieve treatment goals, patients with diabetes must take an active role in their care and manage their condition on a daily, or even hourly, basis. Patient self-management includes adherence to recommendations for medications, dietary intake, exercise, self-monitoring of blood glucose, and risk management practices (e.g., dental, eye, and foot care) along with developing problem-solving skills and healthy coping behaviors.7 Patients require both initial education about their condition and how to manage it as well as ongoing education to fill in knowledge gaps, address challenges as they arise, and support adherence over time.
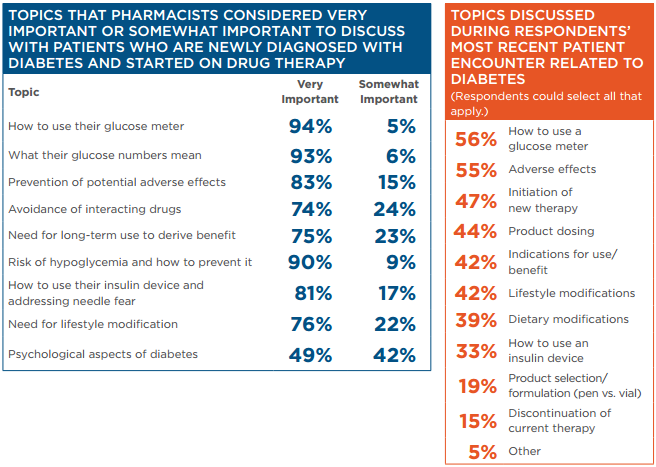
Educating newly diagnosed patients is a crucial component of diabetes care. Diabetes education is important for teaching patients to control symptoms (when present) and to engage in activities that will slow disease progression. Pharmacists responding to the APhA survey identified a variety of topics that they thought were important to discuss with patients who are newly diagnosed with diabetes and started on drug therapy.
Ongoing support and education are also important for promoting persistence to therapy (i.e., adherence over time). Among patients who initially fill their prescriptions, many are not persistent (i.e., they discontinue the medication). For example, an analysis of prescriptions for 238,000 patients with type 2 diabetes found that 47% discontinued their medications over a 1-year period.8
Survey respondents reported being involved in educating patients regarding a wide range of topics and fielding numerous questions from patients about their diabetes care.
Supporting Patient Adherence
 Adherence to pharmacologic therapy has a strong impact on A1C levels and is associated with fewer hospitalizations for patients with diabetes.9,10 Conversely, poor patient adherence to therapy is a major contributor to failure to achieve treatment goals.
Adherence to pharmacologic therapy has a strong impact on A1C levels and is associated with fewer hospitalizations for patients with diabetes.9,10 Conversely, poor patient adherence to therapy is a major contributor to failure to achieve treatment goals.
Improving adherence has been shown to greatly improve outcomes associated with diabetes. For example, an analysis of 135,639 patients with prescriptions for the treatment of diabetes assessed the proportion of days covered over 2 years and then examined hospitalizations and emergency department (ED) use the following year. This study found that patients with diabetes who were not initially adherent to their medications but who improved their adherence had a 13% reduction in the risk of hospitalization or ED visits compared with those who remained nonadherent. On the other hand, patients who were initially adherent and became nonadherent experienced a 15% increase in hospitalization and ED visits compared with those who remained adherent.11 The authors projected that the financial impact of both improving adherence and preventing the loss of adherence could amount to an $8.3 billion annual savings in the United States.11
Factors that influence adherence for patients with diabetes include the patient’s understanding of the treatment regimen and its benefits, adverse events, medication costs, regimen complexity, and the
patient’s emotional well-being.12
Even among patients who are motivated to adhere to their medications, there are many barriers. Patients often face logistical barriers to regularly refilling their medications, or they may be forgetful about their medication use. Multifaceted interventions that include thorough patient education with many touch points over time are necessary to support adherence. Treatment approaches that address the treatment burden (e.g., regimen complexity, hypoglycemia risk, adverse effects) are helpful. Assisting patients in developing a daily routine for taking medication, including the use of pillboxes and reminders, can address forgetfulness. However, factors that affect adherence are highly variable and pharmacists must individualize treatment interventions based on patient needs.12
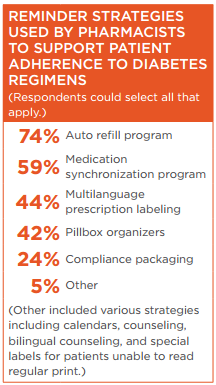
Survey respondents reported that they use a variety of strategies to identify patients who potentially may have issues with their medications and employ reminder strategies to support patient adherence to diabetes regimens.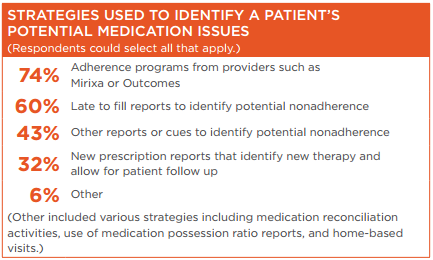
Pharmacists’ Knowledge and Skills to Provide Diabetes Services and Educate Patients About Insulin
The majority of the pharmacists responding to the APhA survey (64%) had earned a PharmD degree, 35% had earned a BS in pharmacy, and 1% had earned an MS in pharmacy. In addition, 62% had attained additional professional credentials.
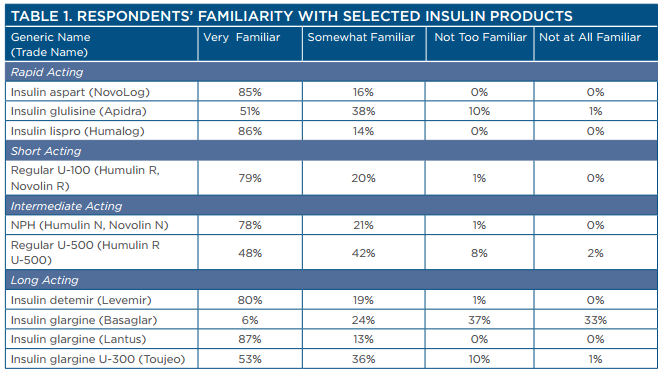
Although many respondents had advanced training, they also were aware of having information gaps related to emerging insulin therapies. Survey respondents reported a high level of familiarity with most insulin products but had some gaps in knowledge, particularly regarding newer products and more concentrated forms of insulin (Table 1).
Pharmacists reported that key factors regarding their decisions to recommend insulin products included efficacy, safety, ease of use, potential to decrease the patient’s medication burden, and cost to the patient. Survey respondents reported that they are likely to receive a variety of questions from patients regarding their insulin use (Table 1).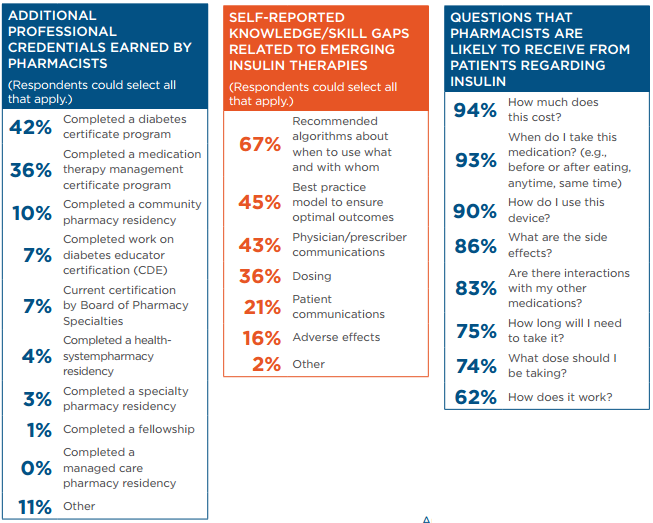
Role of Insulin in the Treatment of Diabetes
Roles of Basal and Prandial Glucose Control
The ADA treatment guidelines recommend initiating insulin treatment with basal insulin. However, they note a growing body of evidence that demonstrates both fasting plasma glucose and postprandial glucose must be managed to optimize patients’ A1C levels.6
Data indicate that postprandial glucose excursions increase oxidative stress and impact the risk for microvascular and macrovascular events.14 However, the relative contribution appears to be affected by the patient’s overall glycemic control. The relative contribution of postprandial glucose is more important in patients who are close to goal and decreases as A1C increases. On the other hand, the relative contribution of fasting plasma glucose appears more important with worsening glycemic control and increases as A1C increases.15,16
Insulin is a mainstay of diabetes treatment. It is the primary pharmacologic therapy for patients with type 1 diabetes, and insulin is often integrated in treatment regimens for patients with type 2 diabetes. Type 2 diabetes is characterized by progressive loss of β-cell function, which leads to worsening glycemic control and usually requires the addition of multiple medications over time. Most patients with type 2 diabetes will eventually require insulin to achieve glycemic control. However, the optimal time to initiate insulin is a matter of debate. Historically, it was one of the last pharmacologic options to be added to treatment regimens. More recent data suggest that early initiation of insulin can help preserve β-cell function, improve insulin sensitivity, and may have a beneficial effect on disease progression.13 Therefore, many clinicians now recommend insulin earlier in the course of treatment for type 2 diabetes.
The 2016 treatment guidelines from the ADA recommend metformin as the initial treatment option for most patients with type 2 diabetes.6 According to these guidelines, if patients are unable to achieve adequate glycemic control, a second agent should be added. Options for the second agent include sulfonylureas, thiazolidinedione, dipeptidyl peptidase 4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists, or basal insulin.6 If glycemic control is not achieved with dual therapy, a third agent from this list should be added. Finally, if triple therapy is inadequate, insulin should be added (if it has not already been initiated). Basal insulin alone is the most convenient insulin regimen; and insulin is typically initiated as a single injection. Targeting postprandial glucose with insulin increases regimen complexity but may provide additional benefits. If patients do not achieve their goal A1C with basal insulin, either prandial (rapid-acting) insulin or a GLP-1 receptor agonist should be added.6
The ADA guidelines recommend that dual therapy should be used as initial therapy in patients who have poor glycemic control at diagnosis (A1C ≥9%), particularly if patients exhibit symptoms of hyperglycemia or catabolism.6 They also indicate that treatment should be initiated with combination basal-bolus insulin for patients with more severe hyperglycemia (A1C ≥10% or blood glucose ≥300–350 mg/dL). In addition, the guidelines recommend that insulin be initiated for patients who do not promptly achieve treatment targets with other therapies.6
A number of patient factors, including patient preference, likelihood of adherence, risk of adverse events (e.g., hypoglycemia), and out-of-pocket costs, must be considered when selecting among treatment approaches. Twice-daily injections with premixed basal-bolus insulins also can be considered. Finally, continuous subcutaneous insulin infusion pumps are an option for carefully selected patients.
Available Insulin Products
Dozens of insulin products are available on the market, including rapid-acting, regular-acting, intermediate-acting, long-acting, and ultra–long acting formulations (Table 2).17-19 Rapid-acting insulins are used with meals to control postprandial glucose and acutely correct hyperglycemia. Long-acting basal insulins have a duration of action of approximately 24 hours and provide continuous glucose control throughout the day. A few newer products (insulin glargine U-300 and insulin degludec) have a longer duration of action and may be referred to as ultra–long-acting insulins. A rapid-acting inhaled insulin product (Afrezza) was approved in mid-2014 but its use has been very limited.
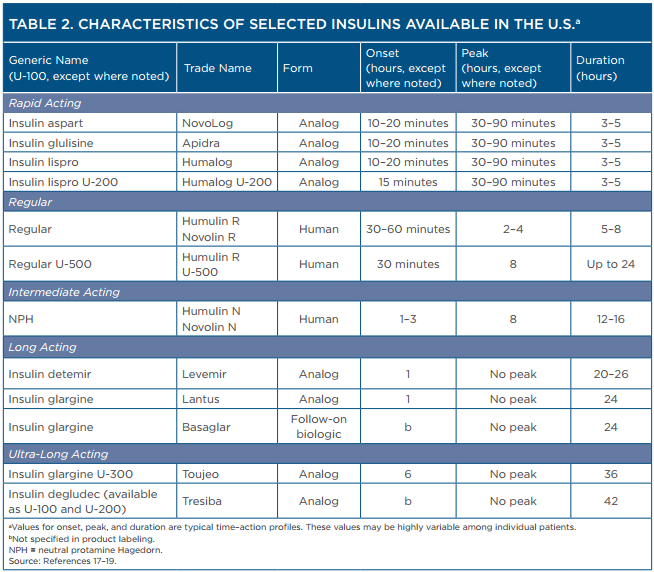
Most insulin products are supplied in U-100 concentrations. However, a few more concentrated products are available, including insulin degludec U-200, insulin lispro U-200, insulin glargine U-300, and regular insulin U-500. These products allow large doses of insulin to be administered with a smaller volume.
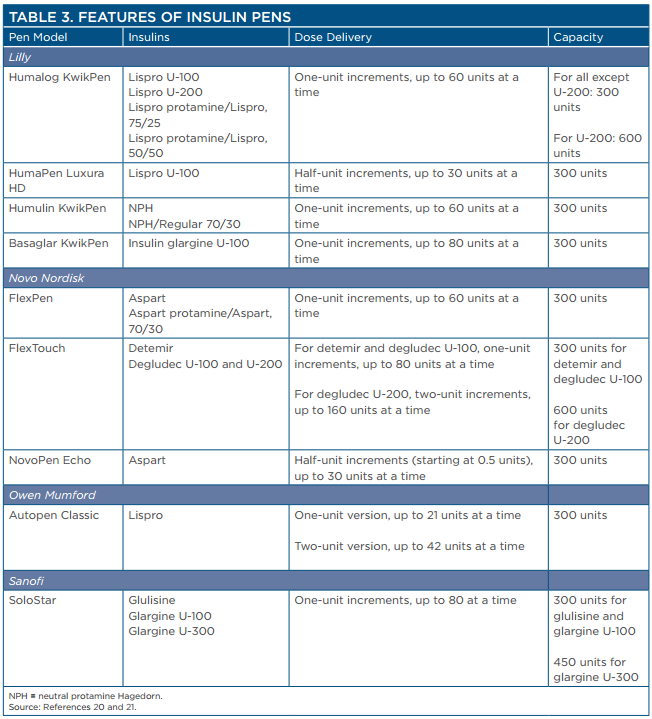
An increasing number of insulins are available for use in insulin pen devices (Table 3).20,21 Pens are convenient to carry and eliminate the need for patients to draw up insulin doses from vials. Insulin pens are considered to provide greater accuracy of dosing and enhance patient adherence. In addition to the products listed in the table, many insulin pen devices are available that contain mixtures of various products to minimize the number of injections for patients.
Considerations When Adjusting Insulin Regimens
The ADA guidelines indicate that treatment should be intensified every 3 months if the patient does not achieve his or her goal A1C. However, in real-world practice, many patients have uncontrolled hyperglycemia for extended periods. (Failure to adjust antihyperglycemic therapy in a timely manner is sometimes referred to as “clinical inertia.”) For example, a retrospective analysis of 12,566 patients whose A1C was not at goal found that patients were treated with metformin alone for a median period of 14 months before therapy was intensified.22 Pharmacists who provide patient care services can address clinical inertia by monitoring patients’ responses to drug therapy and intervening when appropriate.
There is substantial flexibility in treatment recommendations regarding how to intensify regimens when needed. Theoretically, increasing the dosage of insulin can have unlimited efficacy for controlling blood glucose. However, risks of insulin, particularly hypoglycemia and weight gain, must be considered when selecting and titrating dosages and caution must be used when switching among insulin products, particularly those that are more
concentrated.
Patients must closely monitor their blood glucose anytime an adjustment is made to insulin dosages, regimens, or when switching among available insulin products. Changes in insulin concentration, manufacturer, type, or method of administration may affect individual patient responses and require changes in the insulin dose or adjustments to concomitant oral antidiabetic medications. Furthermore, the pharmacokinetics of more concentrated insulin products may be variable. For example, the peak and duration of action of regular insulin U-500 are more similar to those of NPH insulin U-100 than they are to regular insulin U-100.
Products that are currently available in more concentrated forms include regular insulin U-500 (Humulin R U-500), insulin lispro U-200 (Humalog U-200), insulin glargine U-300 (Toujeo), and insulin degludec U-200 (Tresiba). Medication errors in which patients receive the wrong dose are an important safety concern when using more concentrated formulations, particularly if the unit dose must be calculated prior to injection. For example, patients have experienced serious hypoglycemia, sometimes resulting in death, from receiving U-500 insulin instead of U-100 insulin. Patients also have experienced significant hyperglycemia from receiving U-100 insulin instead of U-500 insulin.
Insulin U-500
Regular insulin U-500 (Humulin R U-500) is supplied both in vials and in a pre-filled pen. Patients who use the vial form with a syringe must be carefully trained to ensure that they administer the appropriate dose. Dose conversion is required if patients use vials of insulin U-500 with syringes that are marked for U-100 products. Pharmacists must assess whether patients are capable of administering the correct volume of the U-500 product before dispensing it with U-100 syringes. If U-100 syringes are used, pharmacists should convert the dosing to a U-100 insulin syringe “unit marking” to determine the correct amount of insulin U-500 to draw into the syringe. To determine the correct U-100 insulin syringe “unit marking,” divide the prescribed dose of regular insulin U-500 by 5. For example, if a patient is prescribed 100 units of regular insulin U-500, then the patient should draw up regular insulin U-500 to the 20 “unit mark” on a U-100 insulin syringe (i.e., 100 divided by 5). Patients who use the pre-filled pen do not need to perform a dose conversion.25
Insulin Lispro
Insulin lispro (Humalog) is available in both U-100 and U-200 formulations. The U-100 concentration is available in several forms including vials and a pen. The U-200 concentration is only available in a pen, which is designed to deliver the indicated dose. No dose conversion is required when switching among the pens with the two different concentrations.26 The U-200 product requires fewer pen changes than the U-100 product and may be more convenient for patients who require larger doses of basal insulin.
Insulin Glargine U-300
Insulin glargine U-300 (Toujeo) is available in a pen that delivers the indicated dose; no dose conversion is required.27
Patients who switch to insulin glargine U-300 from a once-daily long-acting or intermediate-acting insulin product should start with the same dose as that being used for the once-daily long-acting dose. To minimize the risk of hypoglycemia when changing patients from twice-daily NPH insulin to once-daily insulin glargine U-300, the recommended insulin glargine U-300 dose is 80% of the total daily NPH dosage.27 Patients who are controlled on insulin glargine U-100 (Lantus) should expect that a higher daily dose of insulin glargine U-300 (Toujeo) will be needed to maintain the same level of glycemic control. If patients switch from Toujeo to Lantus, the dose should be decreased by 20% to prevent hypoglycemia and then titrated based on the patient’s response. Patients should frequently monitor their blood glucose during the first few weeks after switching.27
Insulin Degludec
Insulin degludec (Tresiba) is available in both U-100 and U-200 concentrations. As with insulin lispro and insulin glargine U-300, the pen shows the number of insulin units to be delivered and no conversion is needed. The insulin degludec U-200 pen allows a single injection of up to 160 units at a time and may be more convenient for patients who require large dosages. When switching from another insulin, insulin degludec should be started at the same unit dose as the total daily long-acting or intermediate-acting insulin unit dose.19
Biosimilars and Follow-on Biologics
Because insulin and insulin analogs are biologic products, they may be joined on the market by biosimilar and follow-on biologic insulins when they lose patent exclusivity. Biosimilars and follow-on biologics are biologic products that are highly similar to already approved biologic products. Biosimilar products are not “generic” versions of biologic products; they undergo a different regulatory pathway that accounts for important distinctions between biologics and small molecule drugs. Biosimilar products must demonstrate that they are highly similar to the reference product with no clinically meaningful differences in safety and efficacy.2,3 Biosimilars are expected to be less expensive than the reference drug products, but they are not expected to provide the same degree of cost savings as small molecule generics.
In 2015, Lantus (insulin glargine) was the first insulin analog to lose patent exclusivity, and Basaglar, a new version of insulin glargine, was approved in the United States in December 2015. (Basaglar is considered a follow-on biologic rather than a biosimilar for regulatory reasons.) Many other biosimilar insulins are in development and are likely to enter the market as more patents expire in coming years. The increasing number of insulin products on the market may pose challenges for patients and health care providers, particularly if there are questions regarding how to switch among products.
It is important to recognize that, unlike generic forms of small molecule drugs, biosimilar products cannot be substituted for the reference product by a pharmacist without intervention of the health care provider who prescribed the reference product unless the product is considered to be “interchangeable.” To be considered interchangeable, the sponsor must demonstrate that the biosimilar can be expected to produce the same result in any given patient as the reference product and that there is no increased risk associated with switching back and forth between the biosimilar and reference products.24 As of August 2016, there are no biosimilar products that have demonstrated interchangeability. Patients who switch to biosimilar insulins from a reference product should carefully monitor their blood glucose to assess for any variability in response to the products.
Although Basaglar cannot be automatically substituted for Lantus, patients may be switched among products. If a patient switches to Basaglar from another insulin glargine U-100 product (e.g., Lantus), the dose of Basaglar should be the same as the other insulin glargine product. If a patient switches to Basaglar from an insulin glargine U-300 product (e.g., Toujeo), the recommended initial Basaglar dosage is 80% of the insulin glargine U-300 dosage to reduce risk of hypoglycemia. If changing patients from twice-daily NPH insulin, the recommended initial Basaglar dosage is 80% of the total NPH dosage.18
Addressing Barriers to the Expansion of Diabetes Services
 Although pharmacists’ services have demonstrated benefits for patients with diabetes, there are many barriers that impede pharmacists’ ability to expand their services. Pharmacists responding to the APhA survey reported that lack of payment and issues related to time and workflow were the greatest barriers to expansion of diabetes services. It is likely that greater payment opportunities would allow pharmacists to dedicate more time to the provision of patient care services.
Although pharmacists’ services have demonstrated benefits for patients with diabetes, there are many barriers that impede pharmacists’ ability to expand their services. Pharmacists responding to the APhA survey reported that lack of payment and issues related to time and workflow were the greatest barriers to expansion of diabetes services. It is likely that greater payment opportunities would allow pharmacists to dedicate more time to the provision of patient care services.
Research has demonstrated that pharmacists who practice in settings with more payment opportunities have been able to develop more advanced services.28 These data reveal that addressing payment barriers is crucial for expanding patient access to pharmacists’ services and will allow the health care system to optimize use of existing resources.
Although difficulty obtaining payment remains a barrier for many pharmacists who provide diabetes services in community pharmacy settings, a number of developments are advancing payment opportunities. The health care system is increasingly focused on compensating providers for value rather than volume. Because research demonstrates that pharmacist involvement improves medication adherence, clinical outcomes, and overall cost savings,3 the expanding focus on quality and value is creating new incentives for payers to compensate pharmacists.
On a federal level, changes that support pharmacists’ services include an increasing focus on compensating providers for the quality of care. Many quality measures that have been developed are specific to diabetes, and others, such as those that address adherence, can be applied to diabetes. In many settings, such as accountable care organizations (ACOs), financial incentives are provided for improving quality. This framework creates a financial justification for the ACO to compensate pharmacists for services. Medicare is expanding value-based payment models and alternative payment models (e.g., ACOs) that can improve health care quality and reduce total health care costs. This shift increases accountability for both quality and total cost of care, and it places a greater focus on population health management as opposed to payment for specific services.
In addition, although pharmacists cannot bill Medicare Part B directly as providers, there are other options for pharmacists to receive compensation for their patient care services. For example, pharmacists who provide disease management, medication management, and other patient care services at physicians’ offices can have their services billed using “incident to” codes when specific criteria are met. Other services that pharmacists can indirectly be compensated for by Medicare include transitional care management, annual wellness visits, and chronic care management. Pharmacists may provide these services to patients with diabetes and receive compensation.
Developments on state and local levels also are facilitating payment for pharmacists’ services. More than 30 states have programs that allow payment to pharmacists for the delivery of patient care services, including payment for services provided to Medicaid enrollees, and for the delivery of services to state employees. Some states have more developed payment opportunities than others. For example, while many states consider pharmacists to be providers and allow payment, in 2015, Washington State became the first state to require that third-party payers include pharmacists in their networks and compensate pharmacists for patient care services.28 As these and other changes increase payment opportunities, pharmacists will likely have expanded opportunities to provide diabetes care in community-based practices.
References
- Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172–9.
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Available at: https://www.cdc.gov/diabetes/pdfs/data/2014-report-estimates-of-diabete…. Accessed September 20, 2016.
- Pousinho S, Morgado M, Falcão A, Alves G. Pharmacist interventions in the management of type 2 diabetes mellitus: a systematic review of randomized controlled trials. J Manag Care Spec Pharm. 2016;22:493–515.
- Wong ND, Patao C, Wong K, et al. Trends in control of cardiovascular risk factors among US adults with type 2 diabetes from 1999 to 2010: comparison by prevalent cardiovascular disease status. Diab Vasc Dis Res. 2013;10:505–13.
- Casagrande S, Fradkin JE, Saydah SH, et al.
The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36:2271–9. - American Diabetes Association. Standards of medical care in diabetes—2016. Section 7. Approaches to glycemic treatment. Diabetes Care. 2016;39(suppl 1):S52–S59.
- American Association of Diabetes Educators. AADE7 Self-Care Behaviors. Available at: https://www.diabeteseducator.org/patient-resources/aade7-self-care-beha…. Accessed September 20, 2016.
- Farr AM, Sheehan JJ, Curkendall SM, et al.
Retrospective analysis of long-term adherence to and persistence with DPP-4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31:1287–305. - Rhee MK, Slocum W, Ziemer DC, et al. Patient adherence improves glycemic control. Diabetes Educ. 2005;31:240–50.
- Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–53.
- Jha AK, Aubert RE, Yao J, et al. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff. 2012;31:1836–46.
- Morello CM, Chynoweth M, Kim H, et al. Strategies to improve medication adherence reported by diabetes patients and caregivers: results of a Taking Control of Your Diabetes survey. Ann Pharmacother. 2011;45:145–53.
- Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776–85.
- Madsbad S. Impact of postprandial glucose control on diabetes-related complications: how is the evidence evolving? J Diabetes Complications. 2016;30:374–85.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881–5.
- Kang X, Wang C, Chen D, et al. Contributions of basal glucose and postprandial glucose concentrations to hemoglobin A1c in the newly diagnosed patients with type 2 diabetes—the preliminary study. Diabetes Technol Ther. 2015;17:445–8.
- American Diabetes Association. Insulins Available in the United States. Diabetes Forecast. March/April 2016. Available at: http://www.diabetesforecast.org/2016/mar-apr/images/2016-insulin-chart-…. Accessed September 28, 2016.
- Basaglar (insulin glargine injection) prescribing information. Indianapolis, IN:
Lilly USA; June 2016. - Tresiba (insulin degludec injection) prescribing information. Plainsboro, NJ:
Novo Nordisk; July 2016. - Wahowiak L. Insulin Pens Consumer Guide 2016. Diabetes Forecast. March 2016. Available at: http://www.diabetesforecast.org/2016/mar-apr/product-guide-insuilin-pen…. Accessed September 21, 2016.
- Lilly USA. Basaglar KwikPen Instructions for Use. Available at: pi.lilly.com/us/basaglar-kwikpen-us-ifu.pdf. Accessed September 20, 2016.
- Fu AZ, Qiu Y, Davies MJ, et al. Treatment intensification in patients with type 2 diabetes who failed metformin monotherapy. Diabetes Obes Metab. 2011;13:765–9.
- Heinemann L, Home PD, Hompesch M. Biosimilar insulins: guidance for data interpretation by clinicians and users. Diabetes Obes Metab. 2015;17:911–8.
- Food and Drug Administration. Information on biosimilars. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelope… Applications/Biosimilars. Accessed September 28, 2016.
- Humulin R U-500 (insulin human injection) prescribing information. Indianapolis, IN:
Lilly USA; March 2016. - Humalog (insulin lispro injection) prescribing information. Indianapolis, IN: Lilly USA; November 2015.
- Toujeo (insulin glargine injection) prescribing information. Bridgewater, NJ: Sanofi-Aventis US; September 2015.
- American Pharmacists Association. Pharmacists’ Patient Care Services Digest. March 2016. Available at: http://www.pharmacist.com/digest. Accessed September 21, 2016.
