Focus On Pneumococcal Vaccines for Adults

This resource is designed to offer pharmacists a concise and accurate tool to support making recommendations for administering pneumococcal vaccines to appropriate adult patients. Information is included for addressing questions and concerns so that pharmacists are prepared to educate patients about the available vaccines.
Pneumococcal Disease: A Cause of Serious Illness
Pneumococcal disease is a significant contributor to illness in adults and children; it is caused by Streptococcus pneumoniae, a gram-positive bacterium that colonizes the nasopharynx of up to 90% of healthy people.1 Although carriers of S. pneumoniae may be asymptomatic, they can transmit the bacteria to others through airborne respiratory droplets or by direct contact with infected respiratory secretions.
More than 90 serotypes of S. pneumoniae have been known to cause serious disease, including pneumonia, bacteremia, meningitis, sinusitis, and otitis media.1,2 The 10 most common serotypes are estimated to account for approximately 62% of invasive disease worldwide.1 According to the Centers for Disease Control and Prevention (CDC), an estimated 4 million illness episodes, 445,000 hospitalizations, and 22,000 deaths are attributed to pneumococcal disease annually in the United States.2
Pneumococcal pneumonia is the most common infection resulting from S. pneumoniae, accounting for approximately one-third of community-acquired pneumonias.1 In the United States, approximately 400,000 adults are hospitalized with pneumococcal pneumonia each year.1
Pneumococcal bacteremia is a serious infection of the bloodstream that occurs in 25% to 30% of patients who have pneumococcal pneumonia.1 While the symptoms of pneumococcal bacteremia are usually nonspecific (e.g., fever, chills, difficulty breathing), it can lead to septicemia and cause death in 20% of patients (up to 60% of elderly patients).1,2
S. pneumoniae causes over 50% of all cases of bacterial meningitis, affecting an estimated 3,000 to 6,000 patients annually.1 Pneumococcal meningitis is fatal in approximately 22% of adults.1,3 Signs and symptoms of pneumococcal meningitis are similar to other bacterial causes of meningitis and may include headache, lethargy, irritability, seizures, vomiting, cranial nerve involvement, and coma. Neurologic sequelae (e.g., focal neurological deficits, hearing loss, long-term cognitive deficits) are common in patients who survive a case of pneumococcal meningitis.4
Vaccines for the Prevention of Pneumococcal Disease
Two vaccines are currently available for the prevention of disease caused by S. pneumoniae. One is a 13-valent pneumococcal conjugate vaccine (PCV13) and the other is a 23-valent pneumococcal polysaccharide vaccine (PPSV23):1
- PCV13 (Prevnar 13—Pfizer).
- PPSV23 (Pneumovax 23—Merck).
Who should receive the pneumococcal vaccines, and when?
Both pneumococcal vaccines are recommended for all adults 65 years of age and older, as well as some adults 19 to 64 years of age. To view details and recommended vaccine schedules, go to:5,6 https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/pneumo.html and https://www.cdc.gov/vaccines/schedules/hcp/adult.html.
The recommended uses of PCV13 and PPSV23 in adults are complicated and have undergone several changes in recent years. Careful reading of the Advisory Committee on Immunization Practices (ACIP) immunization schedules and footnotes will help pharmacists determine the recommended uses of these vaccines in various patient populations.6 Key ACIP recommendations are summarized in this brochure.
For adults aged 65 years and older:
- Patients who have not previously received pneumococcal vaccine should receive one dose of PCV13 followed 1 year later by one dose of PPSV23.
- Details for those who have previously received pneumococcal vaccine are shown in Figure 1.
For adults 19 to 64 years of age:
- Figure 2 shows risk groups of patients that should receive pneumococcal vaccine(s) as well as the recommendations for the schedule for administering the vaccine(s).6
General tips for the use of pneumococcal vaccines in adults include the following:6,7
- All adults should receive:
- Only one dose of PCV13, with the age for administration depending on the presence of various health conditions.
- One to three doses of PPSV23, depending on age and health conditions.
- PCV13 and PPSV23 should not be administered concomitantly.
- When both PCV13 and PPSV23 are indicated, PCV13 optimally should be administered first.
- The interval between PCV13 and PPSV23 in immunocompetent individuals should be at least 1 year if given after 65 years of age.
- Among adults with immunocompromising conditions, anatomical or functional asplenia, cerebrospinal fluid leak, or cochlear implant, the interval should be at least 8 weeks.
- The interval between two doses of PPSV23 should be at least 5 years.
Figure 1. Recommendations for Routine Use of Pneumococcal Vaccine for Adults 65 Years of Age and Older
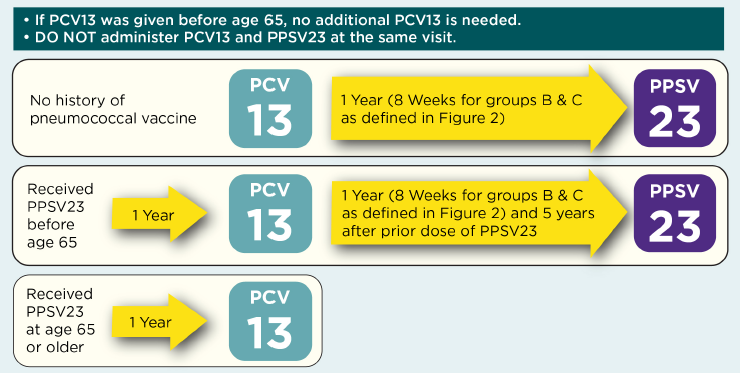
Adapted from Connecticut Immunization Coalition (http://csms.org/wp-content/uploads/2016/01/16-CT-Flu-Algorithm-Yellow-A…) and California Department of Public Health, Immunization Branch (http://eziz.org/assets/docs/IMM-1152.pdf). For further information, see: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/index.html.
Figure 2. Recommendations for Pneumococcal Vaccination of Adults 19-64 Years of Age With High-Risk Conditions
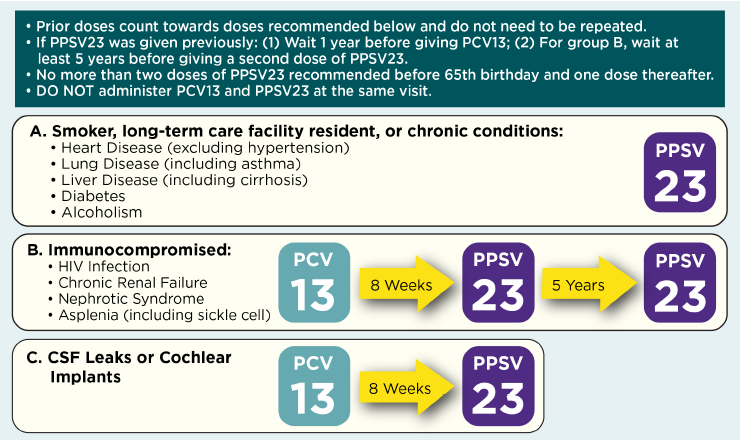
Adapted from Connecticut Immunization Coalition (http://csms.org/wp-content/uploads/2016/01/16-CT-Flu-Algorithm-Yellow-A…) and California Department of Public Health, Immunization Branch (http://eziz.org/assets/docs/IMM-1152.pdf). For further information, see: https://www.cdc.gov/vaccines/vpd/pneumo/hcp/index.html.
Recommend Pneumococcal Vaccine
Pharmacists should have a system in place for identifying patients who are appropriate for pneumococcal vaccine administration and should recommend the vaccine(s) to them. The assessment should utilize information obtained during patient intake as well as any information available from an Immunization Information System (IIS) or the patient’s primary care provider. Examples of ways to prompt vaccine recommendations include:
- Program the pharmacy’s electronic prescription/health record system to identify patients who are 65 years of age or older, and/or have medical conditions indicating a need for vaccination, and trigger an alert if the patient has not previously been vaccinated. The system could also provide prompts to contact the patient for subsequent doses.
- Include a review of vaccination needs during medication therapy management encounters and offer appropriate vaccinations, or refer patients to other providers who can vaccinate, if appropriate.
- Use patient education posters and other resources to increase patient knowledge of the disease, its significance, and availability of vaccines.
- Ascertain whether the patient smokes cigarettes and recommend PPSV23 along with tobacco cessation interventions for appropriate patients.
- Identify patients whose electronic prescription/health records indicate that they are receiving treatment for one of the high-risk health conditions identified in Table 2; those patients could be targeted for educational interventions about the benefits of pneumococcal vaccines.
- Ensure that appropriate disease state management services (e.g., diabetes) have a systematic process in place to assess the need for pneumococcal vaccine and support the administration of appropriate immunizations.
- Utilize technicians and other support personnel and technology to obtain vaccination history, make reminder phone calls to patients, schedule follow-up visits, and educate the public.
Applying the Pneumococcal Vaccine Recommendations
Q: Mrs. Jones is 47 years old and was recently diagnosed with HIV. Assuming she has not previously received any pneumococcal vaccines, which pneumococcal vaccine(s) should she receive, and when?
A: Both PCV13 and PPSV23 are indicated for Mrs. Jones, according to the following schedule:
- She should receive one dose of PCV13 now.
- Because she is immunocompromised, she should receive PPSV23 8 weeks after the administration of PCV13.
- A second dose of PPSV23 should be administered 5 years after the first PPSV23 dose.
- A third dose of PPSV23 should be administered at age 65 years (at least 5 years after the second PPSV23 dose).
Of note, Mrs. Jones will not need PCV13 when she reaches 65 years of age because she will already have received a dose of this vaccine as an adult.
Q: Mr. Vyas is 30 years old and smokes 1 pack of cigarettes per day. The pharmacist recommends that he enroll in a tobacco cessation program, but he remains resistant to change. Which pneumococcal vaccine(s) should he receive, and when?
A: Mr. Vyas is an appropriate candidate for pneumococcal vaccination according to the following schedule:
- One dose of PPSV23 now.
- When he reaches 65 years of age, he should receive one dose of PCV13.
- A second dose of PPSV23 should be administered at least 1 year after the PCV13 (at least 5 years after the first dose of PPSV23).
Answering Patients’ Questions About Pneumococcal Vaccine
Adult patients may be unaware that they are appropriate candidates for pneumococcal vaccine and may have questions. Some potential questions and suggested answers include:3
Why do I need this vaccine?
The CDC recommends pneumococcal vaccine for individuals who are at high risk for developing pneumococcal disease, and because of your age and/or health condition, you are considered high risk. Receiving this vaccine can help prevent serious illness.
 Is pneumococcal disease really that bad?
Is pneumococcal disease really that bad?
Pneumococcal disease can cause serious illness—including pneumococcal pneumonia, meningitis, and blood infection—and death. Recent estimates suggest that 22,000 adults in the United States die from pneumococcal disease each year.
How is pneumococcal disease spread?
The bacteria that cause pneumococcal disease are commonly found in the respiratory tract of healthy adults and are spread from person to person by respiratory droplets in the air or direct contact with infected respiratory secretions. Good hygiene, such as regular hand washing and coughing into your elbow rather than your hand, can help reduce transmission.
I already had a pneumococcal vaccine. Why do I need one again?
There are two different types of pneumococcal vaccines that protect against different types of bacteria that cause pneumococcal disease. The recommendations for the use of these vaccines vary based on your age and health conditions. For example, if you are 65 years or older, you should have one dose of each vaccine. If you are between the ages of 19 and 64 years and have certain health conditions, you may need one dose of PCV13, and one or two doses of PPSV23 before you turn 65 years old.
The maximum number of PPSV23 doses recommended in a lifetime is three doses, which includes up to two doses before 65 years of age for certain high-risk people plus one dose for everyone 65 years of age and older.
Can I get both vaccines at the same time?
No. The administration of the two vaccines should be separated by a minimum of 8 weeks. In some cases, you should wait at least 1 year between both vaccines.
I might be pregnant. Is it safe to get this vaccine?
Both vaccines may be administered to women who are pregnant. The safety of PPSV23 for pregnant women has not been studied, although no negative effects have been reported among newborns whose mothers were inadvertently vaccinated during pregnancy. Women who are at high risk of pneumococcal disease and who are candidates for pneumococcal vaccine should be vaccinated before pregnancy, if possible. ACIP has not published pregnancy recommendations for PCV13 at this time. The use of PCV13 is limited among women of childbearing age.8
Who shouldn’t receive the pneumococcal vaccine?
If you have previously had a severe allergic reaction to the vaccine or one of its components, you should not receive the vaccine. If you currently have a moderate to severe acute illness, you should wait to receive the vaccine until after your condition improves.
If the vaccines are administered to children, why do adults need to receive them too?
One of the pneumococcal vaccines, PCV13, is indicated for all children, and the other, PPSV23, is given to children with certain health conditions. However, these vaccines are relatively new and therefore most adults today did not receive these vaccines when they were children. Current CDC recommendations include vaccinating children and adults.
I can’t remember if I’ve had this the pneumococcal vaccine. What should I do?
We can look in the state’s Immunization Information System (IIS) and check with your primary care provider to see whether you have a record of receiving this vaccine. However, if we cannot locate your records, you should receive the recommended vaccine because extra doses will not harm you.
I’ve already had pneumococcal disease and it was confirmed by a lab test. Do I still need the vaccine?
Yes. There are many types of bacteria that cause pneumococcal disease, and infection with one of them does not provide protection against the others. One of the vaccines protects against 23 subtypes and the second protects against 13 subtypes.
Administer or Refer and Document Pneumococcal Vaccination
When administering pneumococcal vaccine (as well as any other vaccine), documentation regarding vaccine administration and communication with other members of the health care team is essential for providing collaborative care that ensures all the patient’s immunization needs are met. Be sure to:
- Give the patient the current Vaccine Information Statement (VIS) for the vaccine that will be administered.
- Answer any questions the patient may have and discuss expectations with the patient.
- If the patient is willing to be vaccinated and you do not administer the vaccine, provide a referral (written or electronic) to a provider who will.
- Maintain a permanent record of the immunization:
- Date the vaccine was administered.
- Manufacturer of vaccine and lot number.
- Anatomical site of administration and dose.
- Date of VIS and date provided to patient.
- Name, address, and title of the person administering the vaccine.
- Provide the patient with an updated immunization record card.
- Report immunization to the state or local Immunization Information System.
- Send notification of the vaccination to the patient’s primary care provider, if known, and inform the provider that the vaccination will be submitted to the state’s Immunization Information System and the patient’s health insurance, if applicable.
- Report adverse events to the Vaccine Adverse Event Reporting System (VAERS) (https://vaers.hhs.gov) and the patient’s primary care provider.
- If a follow-up vaccination is needed, inform the patient, schedule the vaccination, and/or insert the reminder need within the pharmacy’s reminder-recall system.
Acknowledgments
APhA gratefully acknowledges the financial support from Merck for the development of this resource.

The following individuals served as content and pharmacy immunization practice advisors:
Miranda Wilhelm, PharmD, Clinical Associate Professor
Southern Illinois University Edwardsville, School of Pharmacy
Jessica L. Kerr, PharmD, CDE, Professor
Southern Illinois University Edwardsville, School of Pharmacy
Online Resources
CDC Advisory Committee on Immunization Practices http://www.cdc.gov/vaccines/acip
CDC General Best Practice Guidelines for Immunization http://www.cdc.gov/vaccines/ed/general-recs/index.html
Immunization Action Coalition http://www.immunize.org
Infectious Diseases Society of America Guideline for Vaccination of Immunocompromised Patients
http://www.idsociety.org/Guidelines/Patient_Care/IDSA_Practice_Guidelin…
References
- Centers for Disease Control and Prevention; Hamborsky J, Kroger A, Wolf C, eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Washington, DC: Public Health Foundation; 2015.
- Centers for Disease Control and Prevention. Manual for the Surveillance of Vaccine-Preventable Diseases. 5th and 6th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2012 and 2013. Available at: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html. Accessed June 13, 2018.
- Immunization Action Coalition. Pneumococcus: Questions and Answers. March 2016. Available at: http://www.immunize.org/catg.d/p4213.pdf. Accessed June 13, 2018.
- Lucas MJ, Brouwer MC, van de Beek D. Neurological sequelae of bacterial meningitis. J Infect. 2016;73(1):18-27.
- Centers for Disease Control and Prevention. Pneumococcal ACIP Vaccine Recommendations. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/pneumo.html. Accessed June 22, 2018.
- Centers for Disease Control and Prevention. Recommended immunization schedule for adults aged 19 years or older, United States, 2018. Available at: https://www.cdc.gov/vaccines/schedules/hcp/adult.html. Accessed June 13, 2018.
- Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-7.
- Centers for Disease Control and Prevention. Immunization: You Call the Shots. Pneumococcal. Contraindications and Precautions to Vaccination. Available at: https://www2.cdc.gov/nip/isd/YCTS/mod1/courses/pneumo/10415.asp?student…]. Accessed June 22, 2018.
DISCLAIMER
APhA does not assume any liability for how pharmacists or other health care professionals use this resource.
In all cases, licensed health care professionals must use clinical judgment to ensure patient safety and optimal
outcomes related to pneumococcal immunization.
