Focus On Diabetes

This resource provides a summary for practicing pharmacists on recent developments in the treatment of diabetes. Key updates to recommendations for people with diabetes are highlighted and emerging information about the importance of time in range and glycemic variability are presented.
Diabetes: A Condition on the Rise
The number of individuals in the United States with diabetes has been increasing for decades. The most recent data suggest that 30.3 million people—roughly 9.4% of the population—are living with diabetes. Of those 30.3 million people, approximately 23.1 million have been diagnosed with the condition and 7.2 million are undiagnosed.1 The overwhelming majority (90% to 95%) of individuals with diabetes have type 2 diabetes (T2D) and 5% to 10% have type 1 diabetes (T1D). Additionally, prediabetes affects another 84.1 million adults aged 18 years or older (33.9% of the adult population). Effective interventions are needed to care for these patients and prevent the development of microvascular and macrovascular complications.
2019 Diabetes Standards of Care
The American Diabetes Association (ADA) 2019 Standards of Medical Care in Diabetes (“ADA Standards of Care”) provide guidance for the management of individuals with diabetes. Pharmacologic treatment recommendations are provided for managing both T1D and T2D. The ADA Standards of Care provide a detailed treatment algorithm as well as information about drug-specific and patient factors to consider when selecting antihyperglycemic treatment for adults with T2D.2 These resources are available at diabetesjournal.org. In addition, the American Association of Clinical Endocrinologists (AACE) and American College of Endocrinology (ACE) released an updated comprehensive management algorithm for T2D in 2019.3 This resource is available at aace.com.
Pharmacists should familiarize themselves with the updated treatment algorithms and work to apply emerging information about recommended treatment approaches to the care of people with diabetes.
Selected Pharmacologic Treatment Recommendations
Key pharmacologic treatment recommendations from the 2019 ADA Standards of Care state that most people with T1D should be treated with multiple daily injections of prandial and basal insulin, or continuous subcutaneous insulin infusion. Most individuals with T1D should use rapid-acting insulin analogs to reduce hypoglycemia risk.2
For individuals with T2D, the 2019 ADA Standards of Care recommend metformin as the initial pharmacologic agent. If patients do not achieve individualized glycemic goals with metformin, recommendations for additional medications are based on patient- and medication-related factors, including comorbidities (i.e., atherosclerotic cardiovascular disease [ASCVD] or chronic kidney disease [CKD]), hypoglycemia risk, effects on body weight, side effects, cost, and patient preference as well as other compelling needs based on individual patient factors.
New Recommendations in 20192,4
- Patients with ASCVD who are not at glycemic target despite metformin treatment should be treated with either:
- A glucagon-like peptide 1 (GLP-1) receptor agonist with demonstrated cardiovascular disease (CVD) benefit, or
- A sodium-glucose cotransporter (SGLT2) inhibitor with demonstrated CVD benefit (if the patient’s kidney function is adequate).
- As of April 2019, medications that have demonstrated benefits in cardiovascular outcomes trials include the GLP-1 receptor agonists liraglutide, exenatide extended release, and semaglutide, and the SGLT2 inhibitors empagliflozin and canagliflozin. (Benefits include significant reductions in a composite of major adverse cardiac events.)
- For patients with CKD, an SGLT2 inhibitor with evidence of reducing heart failure and CKD (i.e., empagliflozin, canagliflozin, and dapagliflozin) is the preferred treatment.
- If the SGLT2 inhibitor is not tolerated or if the patient’s kidney function is not adequate, GLP-1 receptor agonists with demonstrated CVD benefit should be used in patients with CKD.
- For individuals who require the greater glucose-lowering effect of an injectable agent, a GLP-1 receptor agonist is preferred to insulin for most patients. This recommendation is based on the lower risk of hypoglycemia, beneficial effects on body weight, and efficacy of GLP-1 receptor agonists compared with insulin in this scenario. Insulin is recommended for individuals whose A1C is above 11%.
Approaches to Glycemic Monitoring and Glucose Management
A1C reflects average glycemia over approximately 3 months and remains the foundation of monitoring a patient’s overall glycemic management. However, A1C does not measure the variability of blood glucose levels throughout the day and does not provide data about how often the patient’s blood glucose is outside the recommended target range.
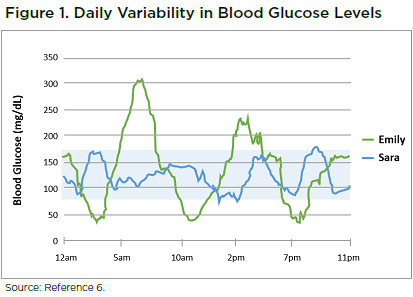 Because glucose management is dynamic, more frequent monitoring provides a more complete picture. As shown in Figure 1, frequent monitoring can reveal that two people with the same average blood glucose levels and the same A1C have very different patterns in glucose levels during the day. A person such as Sara experiences less severe hyperglycemia, less severe hypoglycemia, and less glycemic variability than Emily, even though they have the same average blood glucose levels.6
Because glucose management is dynamic, more frequent monitoring provides a more complete picture. As shown in Figure 1, frequent monitoring can reveal that two people with the same average blood glucose levels and the same A1C have very different patterns in glucose levels during the day. A person such as Sara experiences less severe hyperglycemia, less severe hypoglycemia, and less glycemic variability than Emily, even though they have the same average blood glucose levels.6
Time in Range
To address these gaps in patient data, additional measures such as time in range (TIR) and glycemic variability (GV) are increasingly used to assess the quality of overall glycemic management. TIR refers to the percentage of time that a person’s blood glucose level is within the range of 70 to 180 mg/dL. When there is a greater TIR, for example 70%, this means the person is spending 70% of the time within the target blood glucose range of 70-180 mg/dL. On the contrary, when there is a lesser TIR, for example 30%, this means the person is only spending 30% of the time within the target blood glucose range of 70-180 mg/dL. In any given day, there may be high blood glucose, low blood glucose or blood glucose levels consistently within the target range. These fluctuations throughout the day are referred to as GV.
Researchers have reported that if 50% of a patient’s readings from self-monitoring of blood glucose (SMBG) are in this range, the patient’s A1C should be around 7%.5 Therefore, a patient’s A1C may indicate that his or her diabetes is well managed even when the blood glucose readings are outside the desired range half of the time.
Clearly, individuals with greater GV and less TIR have an increased risk for hypoglycemia. Episodes of hypoglycemia can lead to suboptimal glucose management as they may result in reduction of treatment intensity to minimize hypoglycemia risk. Additionally, hypoglycemia is associated with poor patient satisfaction and worse adherence. Interventions that reduce GV can decrease the risk of hypoglycemia.5
Whether greater GV and less TIR are associated with a greater risk for long-term complications of diabetes has not been proven. Preclinical research suggests that even short periods of high blood glucose concentrations can negatively impact cells and lead to formation of more arterial plaque.6 It has been hypothesized that the excursions of glucose outside the desired range may partially explain why some people experience microvascular complications, while others with the same A1C do not.5 More data are needed to fully understand the impact of TIR and GV on microvascular and macrovascular complications.
Counseling Point: Helping patients understand GV and the daily patterns of their blood glucose may help to identify the causes of variability and empower patients to adjust their behaviors and take an active role in diabetes self-management.
Monitoring TIR and GV
SMBG provides more information about glycemic variability than A1C, but even this approach captures only a few timepoints throughout the day. Continuous glucose monitoring (CGM) and intermittently scanned continuous glucose monitoring (isCGM) are increasingly being recommended for patients to gather additional actionable information about blood glucose levels. These methods provide people with diabetes with more complete information that allows them to see trends in their glucose levels throughout the day and provides information regarding TIR and GV.
The 2019 ADA Standards of Care provide several recommendations related to use of CGM/isCGM:7
- Sensor-augmented pump therapy may be considered for children, adolescents, and adults to improve glycemic control without an increase in hypoglycemia or severe hypoglycemia. Benefits correlate with adherence to ongoing use of the device.
- When prescribing CGM, robust diabetes education, training, and support are required for optimal CGM implementation and ongoing use.
- When used properly, real-time CGM in conjunction with intensive insulin regimens is a useful tool to lower A1C in adults with T1D who are not meeting glycemic targets.
- Real-time CGM may be a useful tool in those with hypoglycemia unawareness and/or frequent hypoglycemic episodes.
- Real-time CGM should be used as close to daily as possible for maximal benefit.
- isCGM use may be considered as a substitute for SMBG in adults with diabetes requiring frequent glucose testing.
SGLT2 Inhibition: A Potential Approach to Improving TIR in T1D
For people with T2D, SGLT2 inhibitors have demonstrated efficacy for improving glycemic management and some have demonstrated that they reduce ASCVD risk (i.e., empagliflozin and canagliflozin) and are renoprotective (i.e., empagliflozin, canagliflozin, and dapagliflozin).4,8 They are currently being studied as an adjunct to insulin in people with T1D.
 In people with T1D, the addition of SGLT2 inhibitors has been found to improve glycemic management (0.4%–0.5% A1C reduction), reduce body weight, and decrease insulin dosage requirements in 24-week trials.8,9 These agents have also been found to reduce GV and improve TIR. For example, a recent clinical study found that sotagliflozin (an investigational SGLT 1/2 inhibitor), when combined with optimized insulin in adults with T1D, significantly increased glucose TIR and reduced 2-hour postprandial glucose levels assessed after a standardized mixed meal, without increasing time spent at <70 mg/dL.10
In people with T1D, the addition of SGLT2 inhibitors has been found to improve glycemic management (0.4%–0.5% A1C reduction), reduce body weight, and decrease insulin dosage requirements in 24-week trials.8,9 These agents have also been found to reduce GV and improve TIR. For example, a recent clinical study found that sotagliflozin (an investigational SGLT 1/2 inhibitor), when combined with optimized insulin in adults with T1D, significantly increased glucose TIR and reduced 2-hour postprandial glucose levels assessed after a standardized mixed meal, without increasing time spent at <70 mg/dL.10
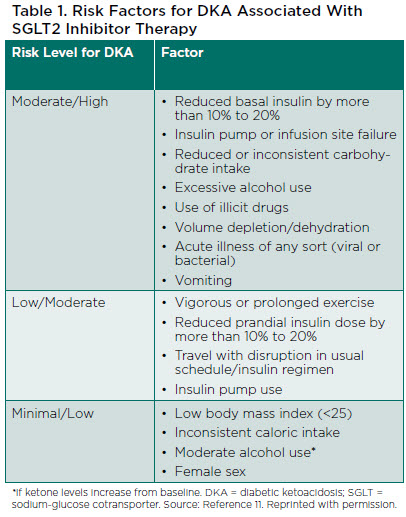
SGLT2 inhibitors provide these benefits without significantly increasing the risk of hypoglycemia. However, they are associated with an increased risk for diabetic ketoacidosis (DKA) and mycotic infections.9 While the risk of DKA is increased, the absolute risk is relatively low and can be minimized with appropriate education and an understanding of patients who are good candidates for use. Risk factors for DKA in people receiving SGLT2 inhibitor therapy are shown in Table 1.11
Use of SGLT2 inhibitors has been associated with postmarketing reports of euglycemic DKA in people with both T1D and T2D. Because the patients in these reports did not have significant hyperglycemia (which is typically seen in patients with DKA), recognition of DKA was delayed.12 It is hypothesized that the occurrence of euglycemic DKA may be a result of non–insulin-dependent glucose clearance, hyperglucagonemia, and volume depletion.12
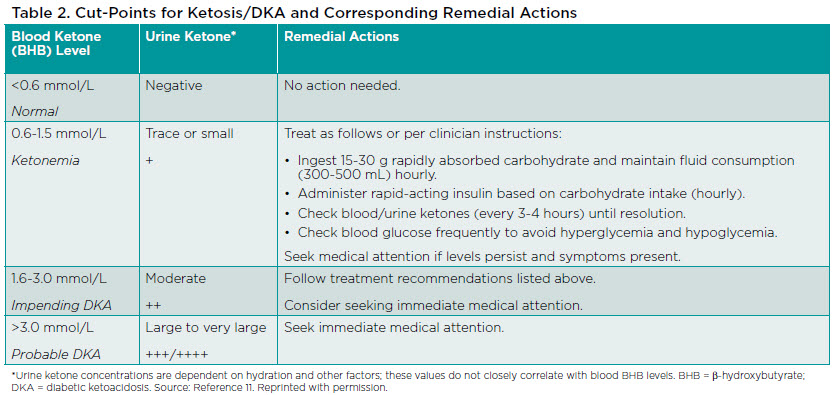
Clinicians should educate patients using SGLT2 inhibitors about the risk for DKA, how to monitor ketones, and symptoms that could indicate DKA. Although individuals with T1D are at greater risk for DKA, education should be provided to all patients. People with either T1D or T2D who experience abdominal pain, nausea, vomiting, dyspnea, or malaise, or develop a metabolic acidosis in the setting of SGLT2 inhibitor therapy should be evaluated for the presence of urine and/or serum ketones, even if they have normal blood glucose levels.11,12 Table 2 lists actions that are appropriate for patients to take in response to various blood or urine ketone values.
In patients who develop ketosis or DKA, rapid-acting insulin should be administered frequently, even if blood glucose levels are not elevated. Foods or liquids containing glucose should be consumed if insulin is administered when blood glucose levels are normal. The STICH protocol is recommended for patients who develop DKA:13
- STop SGLT2 inhibitor treatment for a few days.
- Insulin administration.
- Carbohydrate consumption.
- Hydration with water or noncaloric athletic drink with balanced electrolytes.
Additional data, including longer clinical trials, are needed to further assess the risks and benefits of adding SGLT2 inhibitor treatment for people with diabetes, determine whether there are particular subgroups of people with T1D who would benefit most from SGLT2 inhibitors, and assess the role of SGLT2 inhibitors in the management of T1D.9
RESOURCES FOR SUPPORTING PATIENTS WITH DIABETES
APhA Pharmacist and Patient-Centered Diabetes Care Certificate Training Program
A live educational experience designed to equip pharmacists with the knowledge, skills, and confidence needed to provide effective, evidence-based diabetes care.
Centers for Disease Control (CDC) National Diabetes Education Program
Culturally and linguistically appropriate diabetes education resources for a range of individuals and groups.
American Association of Diabetes Educators
Blood Glucose Monitoring Tip Sheets
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017.
- American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019; 42(Suppl. 1):S90-S102.
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25:69-100.
- Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
- Wright LA, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther. 2017;19(Suppl. 2):S16-S26.
- Yarchoan M. Not all A1Cs are created equal. Available at: https://diatribe.org/not-all-a1cs-are-created-equal. Accessed April 15, 2019.
- American Diabetes Association. 7. Diabetes technology: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl. 1):S71-S80.
- Patoulias D, Imprialos K, Stavropoulos K, et al. SGLT-2 inhibitors in type 1 diabetes mellitus: a comprehensive review of the literature. Curr Clin Pharmacol. 2018;13:261-272.
- McCrimmon RJ, Henry RR. SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia. 2018;61:2126-2133.
- Danne T, Cariou B, Buse JB, et al. Improved time in range and glycemic variability with sotagliflozin in combination with insulin in adults with type 1 diabetes: a pooled analysis of 24-week continuous glucose monitoring data from the inTandem program. Diabetes Care. 2019;42:919-930.
- Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care. February 6, 2019. https://www.ncbi.nlm.nih.gov/pubmed/30728224.
- Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687-1693.
- Garg SK, Peters AL, Buse JB, et al. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther. 2018;20:571-575.
Acknowledgments
APhA gratefully acknowledges the financial support from Sanofi and Lexicon for the development of this resource.
The following individuals served as content advisors:
Kam L. Capoccia, PharmD, BCPS, CDE
Clinical Professor of Community Care Practice
Vice Chair of Practice and Advancement
PGY-1 Community-Based Residency Program Director
College of Pharmacy and Health Sciences
Western New England University
Joshua J. Neumiller, PharmD, CDE, FAADE, FASCP
Vice Chair and Allen I. White Distinguished Associate Professor
Department of Pharmacotherapy
College of Pharmacy and Pharmaceutical Sciences
Washington State University
Disclaimer
APhA does not assume any liability for how pharmacists or other health care professionals use this resource. In all cases, licensed health care professionals must use clinical judgment to ensure patient safety and optimal outcomes.
