Biosimilar Basics For Patients
 What Patients Should Know About Biologics and Biosimilars
What Patients Should Know About Biologics and Biosimilars
Advances in medicine have changed the way many diseases are treated. Increasingly, diseases such as cancer, rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, and even asthma are being treated with biologic medications. Biologic medications are able to target the causes of diseases in ways that were not previously possible, allowing patients to live healthier, more active lives. Today, hundreds of millions of patients worldwide have used biologic medications to treat various diseases and have benefited from the improved results.
This brochure describes biologic medications and a type of biologic known as biosimilars, which are highly similar to biologics but may be less expensive for patients. The information is designed to help you make informed decisions about your health care treatment options.
What is a biologic?
Unlike most traditional small-molecule prescription drugs that are made through chemical processes, biological products are generally made from living cells through complex processes. Examples of biologic medications include proteins (called antibodies) that can bind to specific areas or targets in the body. These targets can include the chemicals our cells use to talk to each other. Some biologics can replace naturally occurring proteins in patients who are unable to make their own. Other biologics can help patients with certain blood disorders or immune system disorders. Before biologic drugs are ready to be used by patients, scientists and doctors spend years developing, researching, and testing them in clinical trials to ensure these drugs are safe and effective. Manufacturing a biologic drug can take months and every part of the process must be carefully checked and controlled. Even small changes in the manufacturing process or environment (such as changing temperatures) can make a big difference in the final product. Because biologics are so complex to develop and manufacture, they are often very expensive.
As shown in Figure 1, biologics are much larger and more complex than small-molecule drugs. Because biologics are so different from small-molecule drugs, the U.S. Food and Drug Administration (FDA) has a separate process for reviewing and approving them.
Figure 1. Size Comparison Between a Biologic Monoclonal Antibody Molecule and an Aspirin Molecule
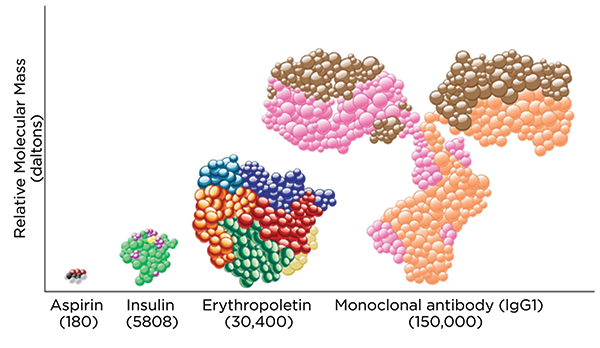
Source: Reference 1. Used with permission.
 What is a biosimilar?
What is a biosimilar?
For many years, generic medicines have been available as less expensive alternatives of small-molecule drugs. Generic medicines are exact chemical copies of brand name drugs and offer less expensive treatment options for patients.
Unlike generic medicines, it is impossible to make an exact copy of a biologic medicine. In fact, all biologic medicines have slight variations from one manufactured batch to another manufactured batch because biologic medicines are derived from living cells, which constantly undergo change. This means that no one batch is an exact copy of another batch. However, it is possible to make a biologic that is highly similar to a biologic product. The original brand name biologic medicine is known as the “reference product,” while the highly similar biologic medicine is called a biosimilar. Biosimilars are still relatively expensive to make. However, like small-molecule generics, biosimilars may provide cost savings to patients and the health care system. One estimate suggests that biosimilars could save the U.S. health care system $250 billion over 10 years.2
Biosimilar medicines can help drive down prices by introducing competition and making medications more accessible to patients, including those who previously may not have been able to afford them.
With approximately $100 billion worth of biologics expected to be off patent by 2020, there is substantial opportunity for biosimilar medicines to create savings for health systems struggling to find the resources to care for a growing and aging global population.
How do I know whether the biosimilar will work the same as the biologic?
 The Biologics Price Competition and Innovation Act, which was enacted as part of the Affordable Care Act of 2010, created a pathway for the FDA to approve biosimilars. The FDA approval process for biosimilar medicines is very thorough, ensuring that all safety and quality standards are met. FDA approval of a biosimilar means that the biosimilar is highly similar to the brand name or reference product. The FDA checks for similar purity, structure, and activity and requires that biosimilars, like the reference products, are studied in patients. This ensures that the reference product and the biosimilar product are clinically equivalent, with no clinically meaningful differences in safety or effectiveness. Since biosimilars are highly similar to the reference product, the FDA allows biosimilar manufacturers to rely on some of the same clinical data that the FDA used to approve the reference product. This means that biosimilar manufacturers can focus on demonstrating that there are no clinically meaningful differences between the biosimilar and the reference product, which the FDA has already determined is safe and effective. As a result, patients can expect to experience the same effects with either a reference product or biosimilar product. Of note, multiple biosimilar products may be approved for a single reference product. The FDA continues to monitor all biologics, including biosimilars, after they are approved and used by a larger number of patients.
The Biologics Price Competition and Innovation Act, which was enacted as part of the Affordable Care Act of 2010, created a pathway for the FDA to approve biosimilars. The FDA approval process for biosimilar medicines is very thorough, ensuring that all safety and quality standards are met. FDA approval of a biosimilar means that the biosimilar is highly similar to the brand name or reference product. The FDA checks for similar purity, structure, and activity and requires that biosimilars, like the reference products, are studied in patients. This ensures that the reference product and the biosimilar product are clinically equivalent, with no clinically meaningful differences in safety or effectiveness. Since biosimilars are highly similar to the reference product, the FDA allows biosimilar manufacturers to rely on some of the same clinical data that the FDA used to approve the reference product. This means that biosimilar manufacturers can focus on demonstrating that there are no clinically meaningful differences between the biosimilar and the reference product, which the FDA has already determined is safe and effective. As a result, patients can expect to experience the same effects with either a reference product or biosimilar product. Of note, multiple biosimilar products may be approved for a single reference product. The FDA continues to monitor all biologics, including biosimilars, after they are approved and used by a larger number of patients.
Biosimilars are relatively new. The first biosimilar in the United States was approved in 2015. However, they were approved earlier in Europe, starting in 2006. Since that time, more than 35 biosimilars have been approved in Europe. To date, biosimilars have been found to be as safe and effective as the reference biologics.3 More than 90 studies have been published that show switching from a biologic to a biosimilar is safe and effective.4 In addition, some national health care provider organizations have published statements supporting the use of biosimilars to improve access to these therapies that otherwise would not have been available to patients due to cost.5
Will my pharmacist substitute the less expensive biosimilar for a biologic in the same way that he or she could do with a generic?
 Not currently. Although biosimilar medicines are like generics in some ways, they cannot be automatically substituted. You must have a prescription specifically written for a biosimilar product in order to receive it. However, pharmacists can communicate with you and your doctor about whether there is a biosimilar available and whether this is a good option for you.
Not currently. Although biosimilar medicines are like generics in some ways, they cannot be automatically substituted. You must have a prescription specifically written for a biosimilar product in order to receive it. However, pharmacists can communicate with you and your doctor about whether there is a biosimilar available and whether this is a good option for you.
Often, a biosimilar will have a lower out-of-pocket cost, although your insurance coverage may affect how much money you might save. Talk to your doctor or pharmacist to see if there are biosimilar options available. While biosimilars are not yet available for all biologics, several are currently available and many more are being developed.
In the future, pharmacists may be able to automatically substitute a biosimilar, similar to the way pharmacists are able to substitute small-molecule generic medicines. The Affordable Care Act created a special category of biosimilars called “interchangeable
biologics.” These interchangeable biosimilars may be substituted for a reference biologic. The FDA is currently working on identifying the additional criteria that will be required for a biosimilar to be considered an interchangeable biologic. Until interchangeable biologics become available, a doctor must specifically prescribe a biosimilar in order for a patient to receive it.
Regardless of whether you are using a biosimilar or the biologic reference product, it is important to know the name of the specific product that you are taking. Biosimilars can be referred to by three different names: the brand name, the core scientific name along with the manufacturer’s name, and the core scientific name followed by a random four-letter suffix. For example, a biosimilar might have the following names: Imagine (brand name), imaginarymab Medmaker (core scientific name with manufacturer’s name), and imaginarymab-gxrm (core scientific name followed by a random four-letter suffix). Any of the names for a biosimilar can be used, but most people find it easiest to use the brand name. Additionally, your pharmacist should keep a record of the manufacturer’s lot number and your medicine’s National Drug Code (NDC). The NDC is unique to each product and can easily identify between the reference product and the biosimilar or among multiple biosimilars. These are important because the reference biologic and all biosimilars for that product share the same core scientific name. There can be multiple biosimilar medicines for the same reference biologic. For example, a reference biologic called imaginarymab could have two biosimilar products called imaginarymab-gxrm and imaginarymab-ltsr. Currently approved biologics will be renamed so that they will also include a four-letter suffix. Make a note of the exact product you are taking so that you can be clear when talking to members of your health care team about your medicines.
Acknowledgments
APhA gratefully acknowledges the financial support from the Biosimilars Council, a division of the Association for Accessible Medicines, for the development of this resource.
The following individual contributed toward the content development and served as the pharmacy practice advisor for this resource:
James G. Stevenson, PharmD, FASHP
Professor, Department of Clinical Pharmacy
University of Michigan College of Pharmacy
References
- Mellstedt H. Clinical considerations for biosimilar antibodies. EJC Suppl. 2013;11:1–11.
- Miller S. The $250 Billion Potential of Biosimilars. Express Scripts website. April 23, 2013. Available at: http://lab.express-scripts.com/lab/insights/industry-updates/the-$250-b…. Accessed May 23, 2018.
- European Medicines Agency. Biosimilars in the EU: Information Guide for Healthcare Professionals. April 2017. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Leaflet/2017/05/WC…. Accessed May 23, 2018.
- Cohen HP, Blauvelt A, Rifkin RM, et al. Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78:463–78.
- British Society of Gastroenterology. BSG Guidance on the Use of Biosimilar Infliximab CT-P13 in Inflammatory Bowel Disease. February 2016. Available at: https://www.bsg.org.uk/resource/bsg-guidance-on-the-use-of-biosimilar-i…. Accessed May 23, 2018.
Disclaimer
APhA does not assume any liability for how pharmacists or other health professionals use this resource. In all cases, licensed health professionals must use clinical judgment to ensure patient safety and optimal outcomes related to biologic medications.
